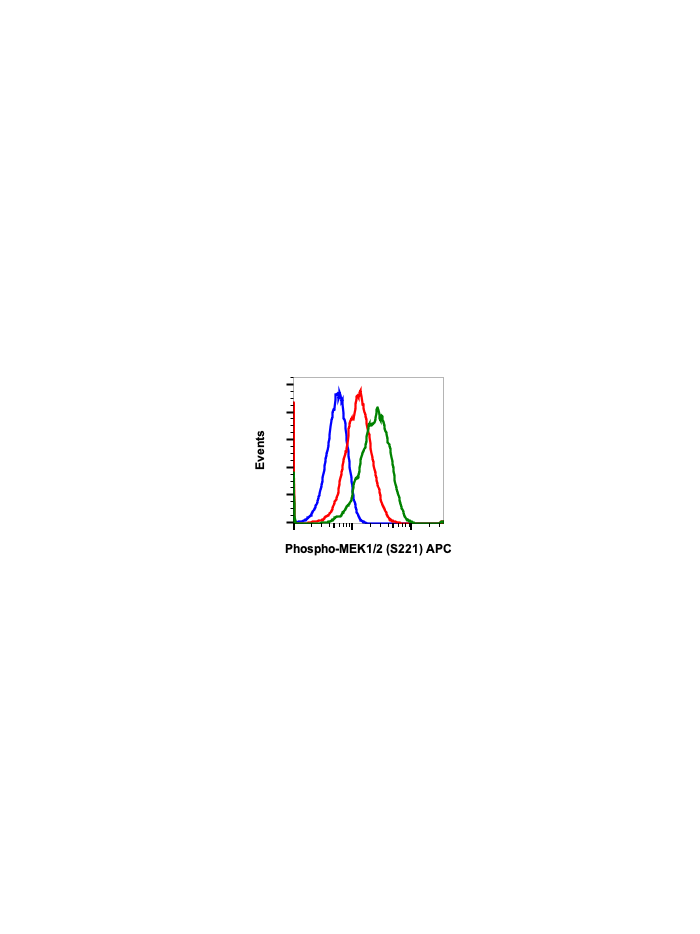
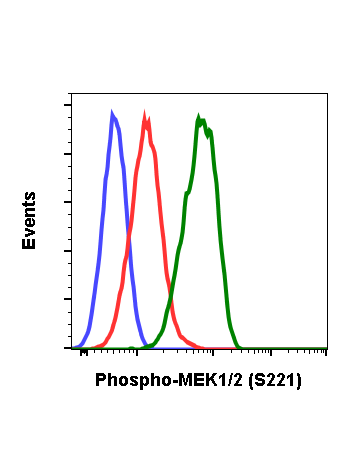
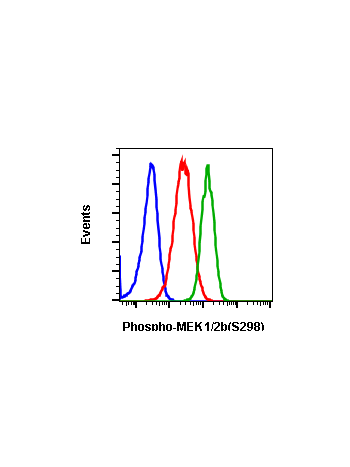
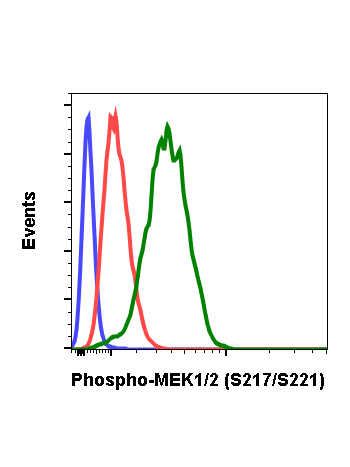
Phospho-MEK1/2 (Ser221) (D3) rabbit mAb APC Conjugate
Mitogen-activated protein kinase (MAPK) is the main building block of the intracellular signaling network. The signals are initiated by activation of a small G protein (e.g., Ras), followed by a sequential activation of several sets of protein kinases. The extracellular signal-regulated kinase (ERK) pathway is stimulated by a large number of extracellular stimuli as well as various internal processes. This cascade regulates processes related to proliferation, differentiation, development, and oncogenic transformation. The signaling is usually initially started by activation of small G proteins (e.g. Ras), which in turn recruits to the membrane and activates the MAP3K (Raf kinase). The cascade leads to the activation of MAP kinase kinases (MEKs). MEKs are phosphorylated at Ser218 and Ser222 in MEK1. The phosphorylation activates MEKs. Phosphorylation of MEK at other sites regulates its activity as well. For example, phosphorylation at Ser386 by ERKs may either inhibit ERKs activity or under certain condition, facilitate the activation of ERKs by enhancing the MEK1 binding to Grb10 scaffold protein. Phosphorylation of MEK1 at Ser298 by p21-activating protein (PAK1) may lead to its activation whereas this can be inhibited by a feedback phosphorylation of MEK1 at Thr292 by ERKs. Protein Ser/Thr phosphatase inactivates MEK by dephosphorylation of pSer218 and pSer222. Other phosphatases my also regulate MEK activity. Upon activation, MEKs behaves as a dual kinase and phosphorylate key Tyr and Thr residues of ERKs, thus causing their activation.
| Applications | Flow Cytometry |
|---|---|
| Clone | MEK12S221-D3 |
| Format | APC |
| Validated Reactivity | Human, Mouse |
| Cross Reactivity | Predicted to work with mouse, rat and other homologues. |
| Clonality | Monoclonal |
| Immunogen | A synthetic phospho-peptide corresponding to residues surrounding Ser221 of human phospho MEK1/2. |
| Formulation | 1X PBS, 0.09% NaN3, 0.2% BSA |
| Isotype | Rabbit IgGk |
| Preparation | Protein A+G |
| Recommended Usage | For flow cytometric staining, the suggested use of this reagent is 5 µL per million cells or 5 µL per 100 µL of staining volume. It is recommended that the reagent be titrated for optimal performance for each application. See product image legends for additional information. |
| Storage | 2-8ºC |
| Pseudonyms | Dual specificity mitogen-activated protein kinase kinase 1/2, MAPK/ERK kinase 1, MAPKK1, MAPKK2, MAP2K1, MAP2K2, PRKMK1, PRKMK2 |
| Uniprot ID | Q02750 P36507 |
| References | Raman M. and Cobb MH., (2003) Curr Biol 13:R886-888. Kuida K., and Boucher DM. (2004) J Biochem 135:653-656. Torii S., et al., (2004) J Biochem, 136:557-561. Yoon S. and Seger R., (2006) Growth Factors 24:21-44. Natel A. et al., (1998) J Biol Chem 273:10475-10484. |